Table of contents
1. Introduction
2. Understanding HPV
3. Impacts of HPV on the Body
4.
5. Diagnosis & tests
6. Prevention and vaccination
7. Debunking Myths about HPV
8. Resources
Written by Kalina Mihaylova
Medically reviewed by Sarah Montagu (RN DFSRH, BSc)
Illustrated by Maria Papazova
Introduction
Human papillomavirus (HPV) is a virus that infects the cells in the skin (the outer layer of your body) and the mucosa (the inner lining of organs and body cavities, such as your mouth, vagina and nose).
The infection is incredibly common - about 8 in 10 people will get HPV at some point in their life. Despite the fact that most people, both men and women, will end up with this infection, actionable information about its management and the cause of complications, such as cervical and oral cancers, is lacking.
We believe that people shouldn’t need a medical degree to access clinical research and breakthroughs in healthcare. This guide aims to consolidate all evidence-based information about HPV into an easy-to-read digestible format.
Understanding HPV
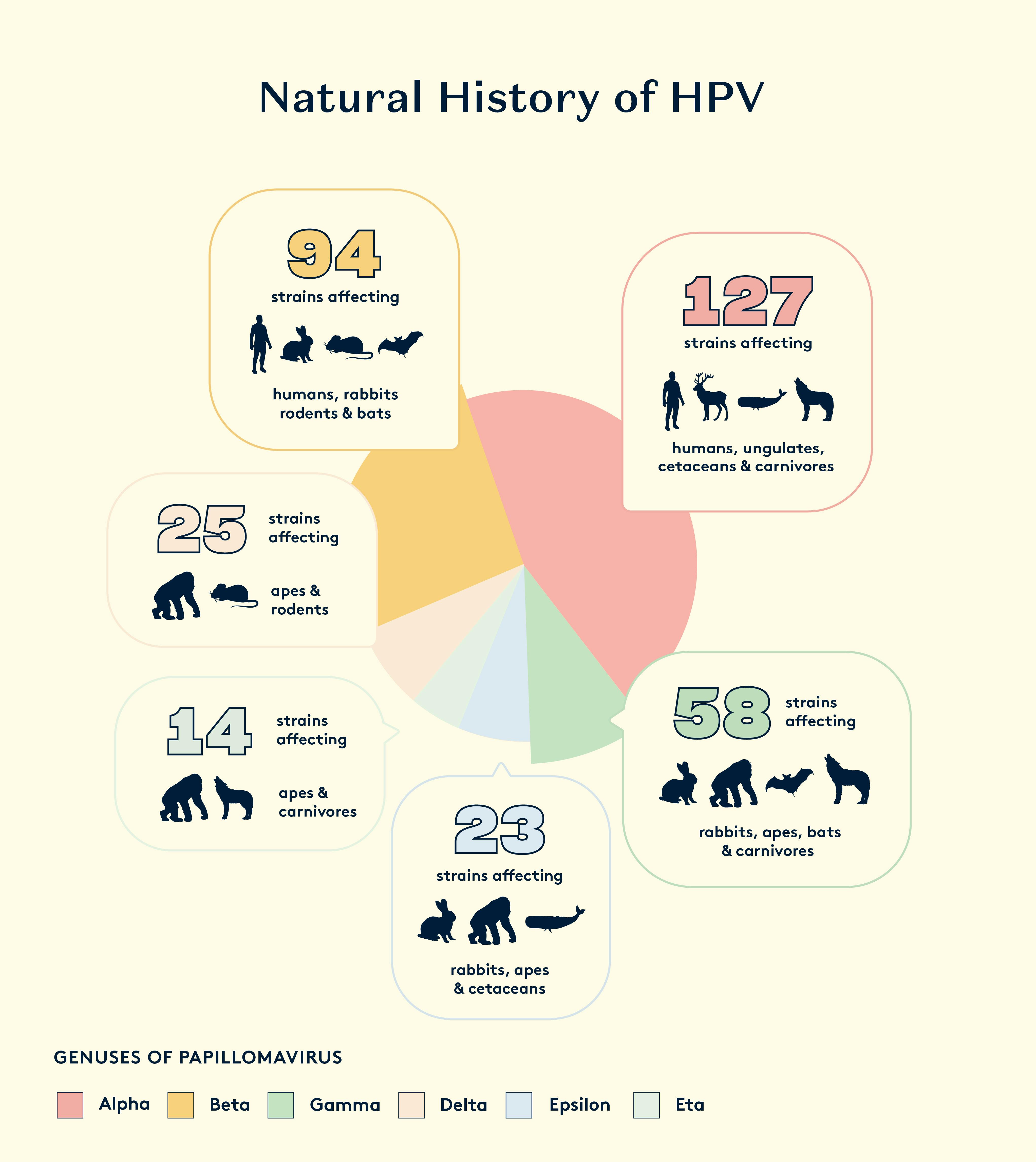
Papillomavirus is one of the oldest DNA viruses known. Evidence shows that it emerged 450 million years ago. More than 200 different strains have been identified that infect humans, also referred to as Human Papillomavirus (HPV).
Papillomaviruses have been found to be the key to cervical cancer and some other important cancers within the last 40 years. However, modern research into the wide range of different papillomavirus strains and slow mutation rate has led to the conclusion that this virus can be traced back for several hundred million years. It is therefore likely that cervical cancer, unlike cancers that are related to risk factors of the modern lifestyle, has been an important disease of women from prehistory.
What is HPV?
HPV stands for Human Papillomavirus, which is a group of related viruses. It is the most common sexually transmitted infection (STI) worldwide, infecting over 14 million individuals each year and impacting 80% of sexually active people at some point in their lives. HPV infects the tissue that lines surfaces in our body, the epithelia. This includes the skin and mucosa of the respiratory and genital tracts.
Once it enters the body, the HPV virus needs to replicate and go through numerous defence systems to actually cause harm. In 90% of cases it fails and the infection is cleared naturally by our bodies within two years. In the remaining 10% of cases, the virus develops into a long-term persistent infection that may actually cause harm and potentially progress into cancer. This is a very slow process, usually taking more than 5 years from the time of infection.
It is also possible for some HPV infections to remain latent in the body, without causing any harm.
While HPV can’t be treated, there are things that can be done to minimise the impact of the virus on our bodies. One way to think about prevention is to strengthen your body’s natural defences. That way, even if you get infected, the virus may not end up causing any harm and it can even clear on its own in a couple of weeks or months.
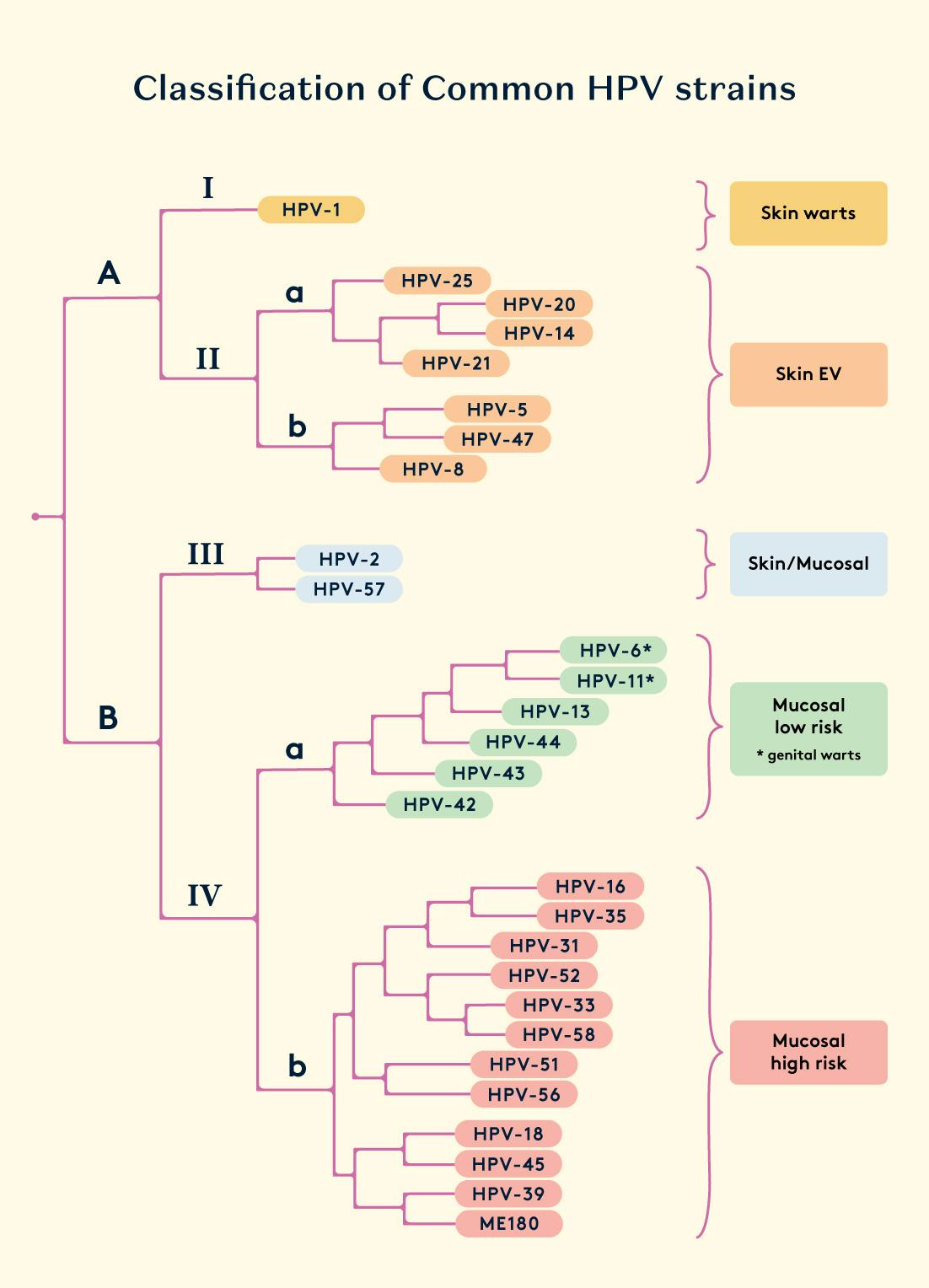
Types of HPV
There are more than 100 types of HPV. Out of these, some are able to infect mucosal membranes, such as the lining of the vaginal tract or the inside lining of the mouth and others are able to infect the skin.
The mucosal HPV types are most commonly transmitted through sexual contact, mother-to-newborn transmission and skin-to-skin contact of the genitals. These strains further classified as low-risk HPVs (LR-HPVs) or high-risk HPVs (HR-HPVs) based on their ability to cause cancer. Persistent infections with a high-risk HPV strain account for 5% of cancers in humans, including almost all cases of cervical cancer, and some cases of the anus, penis and oral cancers. Although most infections are asymptomatic, genital infection by low-risk HPV is associated with genital warts.
Skin HPV types are incredibly common - 90% of healthy people have these. They are transmitted through skin-to-skin contact. Studies show that family members usually share identical skin HPV strains. Skin HPVs are inclined to infect the cells of hair follicles, where they can cause persistent infection. Most of them are harmless and some cause skin warts. Some HPVs typically lead to the development of benign tumours such as common warts (verruca vulgaris) and papillomas. Certain types of skin-infecting HPV seem to contribute to the beginning stages of skin cancer development by worsening the effects of UV radiation on the skin, causing DNA damage and genetic changes. It's like they assist in the process rather than directly causing non-melanoma skin cancer.
Transmission
Genital human papillomavirus (HPV) infections are primarily transmitted through sexual contact with an infected individual, typically via sexual intercourse. The risk of exposure is directly related to the number of sexual partners, the introduction of a new sexual partner, and the sexual history of any partner.
The use of condoms can reduce, but does not completely eliminate, the risk of sexual transmission of HPV. In addition to sexual transmission, non-sexual routes of HPV transmission can include vertical transmission from an infected mother to her newborn child.
It is estimated that approximately 80% of people will contract HPV at some point during their lifetime.
Risk Factors
Several risk factors contribute to the acquisition of HPV. One significant factor is engaging in unprotected sex, including vaginal, anal, or oral sex, with an infected partner. The virus is highly contagious, and individuals with multiple sexual partners or those who initiate sexual activity at an early age are at an increased risk.
Lack of vaccination against HPV also heightens susceptibility, especially considering the vaccine's effectiveness in preventing infection with certain high-risk HPV types.
Additionally, a weakened immune system due to conditions such as HIV/AIDS or immunosuppressive medications increases the likelihood of persistent HPV infections.
Tobacco use, particularly smoking, has been identified as a risk factor, as it compromises the immune system and may contribute to the progression of HPV-related lesions.
Finally, a history of sexually transmitted infections (STIs) or Bacterial Vaginosis (BV) may elevate the risk of a persistent HPV infection, as they compromise the ability of the immune system to clear the virus. Learn more in the section HPV and The Vaginal Microbiome.
Mechanism of an HPV infection
Understanding how the DNA of the Human Papillomavirus (HPV) works helps us understand how the virus is able to remain in our bodies for a long time. Think of the DNA as a set of instructions, like buttons on a remote control. In high-risk HPV types, there are special buttons, or genes, called E1, E2, E4, E5, E6, and E7. When these genes are activated, they change the structure of skin or vaginal cells, making them the perfect home for the virus to multiply and overpower the body's defences. When pressed together, these buttons initiate an organised attack towards causing a persistent HPV infection.
To initiate an infection, an HPV viral particle must gain access to the basal cells of the deep layer of the skin or mucosal tissue. This can occur through an abrasion or disruption in the protective barrier of the mucosa.
Once inside the cell, the virus makes its way into the nucleus, the control centre of the cell, and takes control, starting the process of producing more infected cells. As these cells replicate, a pool of HPV-infected cells is established, which is a key milestone in the development of a persistent infection. As the infected cells continue to divide and move towards the outer surface layer, rapid amplification of the virus occurs.
The immune system tries to fight off the HPV infection in a few ways. It tries to detect the foreign viral DNA, triggers inflammation, and produces substances called antiviral cytokines to combat the virus.
However, the high-risk strains of HPV have found ways to evade the immune system. Certain HPV genes can block the sensors that would normally detect the viral DNA inside the infected cells. They can also interfere with the signals that would activate the immune system's response.
However, the high-risk HPV (hr-HPV) genes, such as E5, E6, and E7, work to conceal the infection from the immune system. These genes block the sensors that detect viral DNA in the infected cells, and they also obstruct the signalling pathways of the immune system, thereby regulating the immune response.
HPV and the Vaginal Microbiome
Recent research suggests that the natural communities of bacteria living in the vagina, called the vaginal microbiome, may play an important role in helping the body get rid of HPV infections on its own. The microbiome may also help reverse the development of cervical cancer caused by HPV.
The key players in a healthy vaginal microbiome are a group of bacteria called Lactobacilli. There are four main Lactobacilli strains typically found in a healthy vagina: Lactobacillus crispatus, L. iners, L. jensenii, and L. gasseri. These Lactobacilli bacteria are recognized as the main regulators of a balanced and healthy vaginal environment.
In other words, the presence and balance of these Lactobacilli bacteria in the vagina seem to be important factors in allowing the body to naturally clear HPV infections and potentially even stop the progression to cervical cancer in some cases.
The Lactobacilli bacteria in a healthy vaginal microbiome play an important role in preventing infections. They do this by producing natural antimicrobial substances like lactic acid and hydrogen peroxide, which inhibit the growth of harmful bacteria. Studies have found that people with higher levels of the Lactobacillus crispatus strain in their vaginal bacteria tend to be more successful at naturally clearing HPV infections on their own.
Lactobacillus bacteria also prevent the growth of bacteria that cause bacterial vaginosis (BV), like Gardnerella vaginalis. BV can be beneficial for HPV to persist in the body. Additionally, substances produced by the Lactobacillus crispatus strain, called peptidoglycans, can activate certain immune cells in the cervix that are linked to clearing HPV.
Research has shown that women with BV are more likely to have persistent HPV infections. This is because the bacteria that cause BV, like Gardnerella vaginalis and Prevotella, produce enzymes, called sialidase, that break down the protective barriers in the vaginal area. This allows the HPV virus to more easily reach the deeper layers of mucosa.
Certain other bacteria, like Sneathia species, are commonly found in people with both BV and HPV. These bacteria can activate inflammatory pathways and interfere with the body's immune response, which may contribute to the development of cancer.
On the other hand, a substance called Candin produced by the Candida fungus has been linked to a lower risk of HPV infection. Candin has been shown to stimulate the growth and activity of T cells, which are important immune cells for recognizing and attacking virus-infected cells. This is why it is believed that Candida may potentially help make HPV vaccines more effective as a therapeutic.
Considering the key role of the vaginal microbiome in influencing cervical immune responses, there is a potential for novel treatment pathways that support the clearance of HPV infections by reconstructing the balance of bacteria in the vaginal canal.
Impacts of HPV on the Body
Genital warts
Genital warts, scientifically known as condylomata acuminata (CA), are a prevalent sexually transmitted infection caused by certain types of the human papillomavirus (HPV). These warts typically manifest as growths or lumps in the genital and anal areas and are one of the most common forms of sexually transmitted diseases. In the United States alone, an estimated 500,000 to one million new cases are diagnosed each year.
HPV types 6 and 11 are primarily responsible for the development of genital warts, contributing to approximately 90% of cases. These warts are highly contagious and can be transmitted through sexual contact with an infected person, even if they do not display visible warts. Genital warts may appear as small, flesh-coloured or greyish swellings with a cauliflower-like appearance.
The prevalence of HPV infection has consistently increased over the past 35 years, with an estimated 20 million people in the United States currently affected. This rise is often attributed to both an earlier onset of sexual activity and an increase in the overall number of sexual partners. As a result, almost half of these new infections occur in young adults aged 15 to 24 years. HPV is highly contagious, primarily transmitted through oral, anal, and genital sexual contact.
Engaging in sexual activity with an HPV-infected individual carries a 75-percent chance of contracting the virus and developing genital warts (CA), contributing to a significant 50-percent lifetime risk of acquiring external genital warts (EGW) among sexually active individuals.
Additional risk factors include unprotected intercourse, oral contraceptive use, a history of sexually transmitted infections, smoking, or immunosuppression,.
When it comes to treating genital warts,the focus is mainly on removing the visible warts rather than getting rid of the underlying virus completely. There are different treatment options available, but no single best way to do it. These treatments can vary a lot in terms of cost, side effects, how often you have to take them, how long the treatment lasts, and how well they work.
Some common treatments include applying creams like podophyllotoxin or imiquimod, using destructive methods like trichloroacetic acid or cryotherapy, or surgical procedures like electrosurgery or laser therapy,,,. Each person might get a different treatment depending on their specific situation. Some older treatments, like podophyllin or interferon, are not recommended much anymore because they don't work as well or may have side effects. Researchers are still looking for better ways to treat genital warts more effectively.
Cancer
Together, the HPV-related cancers represent 630,000 new cancer cases per year, which account for ~30% of all cancers caused by infectious agents.
We now know that HPV causes problems in more areas than just the cervix, vagina, and vulva. It can also lead to issues like abnormal cell growth in the anal region in both men and women, and cancers in the penis, scrotum, and throat. However, most of the research has mainly focused on preventing cervical cancer.
Cancer develops silently over many years without showing any signs or symptoms initially. A few cells in a tissue might start changing their genetic makeup, and over time, they undergo more genetic changes during cell renewal. These alterations help the cells avoid detection by the immune system, promote their replication, and ensure their survival. The process of tissue damage and healing contributes to the possibility of genetic damage. The long period, sometimes spanning several decades, between the early stages and the appearance of clinical disease makes it challenging to pinpoint the exact causes of cancer development.
Looking back, the discovery of HPV genomes in cervical cancers and the confirmation of their cancer-causing abilities in the 1990s and beyond revealed that HPV is the main culprit behind cervical cancer and certain cancers in both males and females. Papillomavirus infections can introduce viral genes into cells, which may become part of the cancer cells' genetic makeup. These viral genes have clear cancer-causing properties and largely explain the complex biology of these cancers caused by a virus that cannot reproduce once integrated into the genome. From the virus's perspective, these genetic changes are just chance interactions between viral and human genes. For humans, we can prevent this by vaccinating against HPV to block primary infections. Additionally, there's potential to treat HPV-related lesions by finding ways to deactivate the expression or function of the viral E6 and E7 oncogenes in cells on their way to cancer or even earlier, by selectively eliminating cells with primary viral infections before they become cancerous.
Cervical cancer
Cervical cancer is a common and serious health threat for women. It ranks as the fourth most common cancer in women globally. In 2018, the World Health Organization reported that it was the second most common cancer in less developed regions, with about 570,000 cases and 311,000 deaths. Unfortunately, over 85% of these deaths occurred in those less developed regions. In the UK, approximately one third of women die within five years of the diagnosis of invasive cervical cancer (National Statistics, 2011).
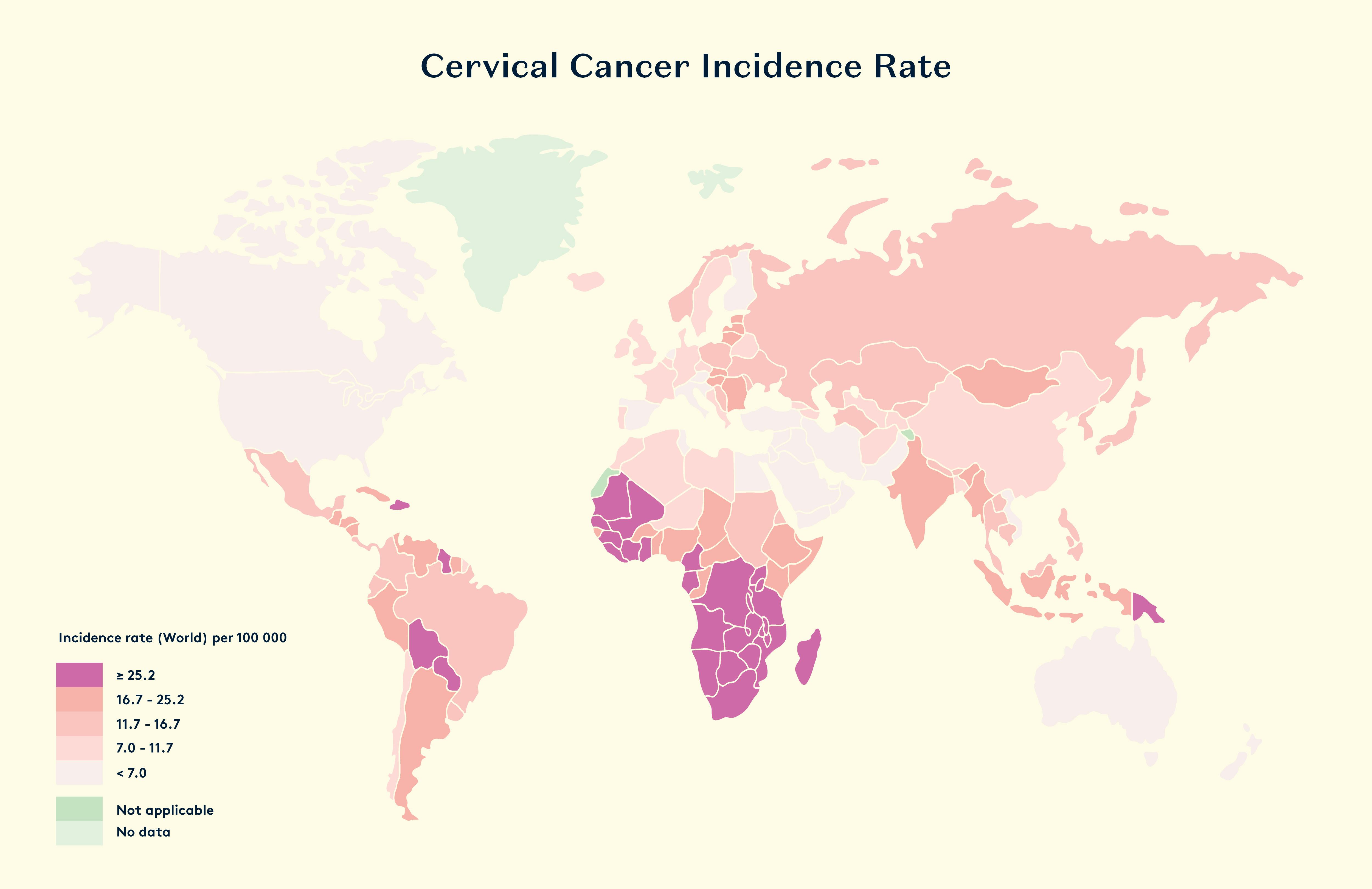
Women who end up with cervical cancer may test positive for a high-risk HPV type 3–5 years before the cancer develops. Having a persistent high-risk HPV infection increases the chances of developing high-grade cervical intraepithelial neoplasia (CIN) and cancer. The two main types of HPV, HPV16 and HPV18, are the most common culprits, responsible for 70% of cervical cancers and precancerous lesions.
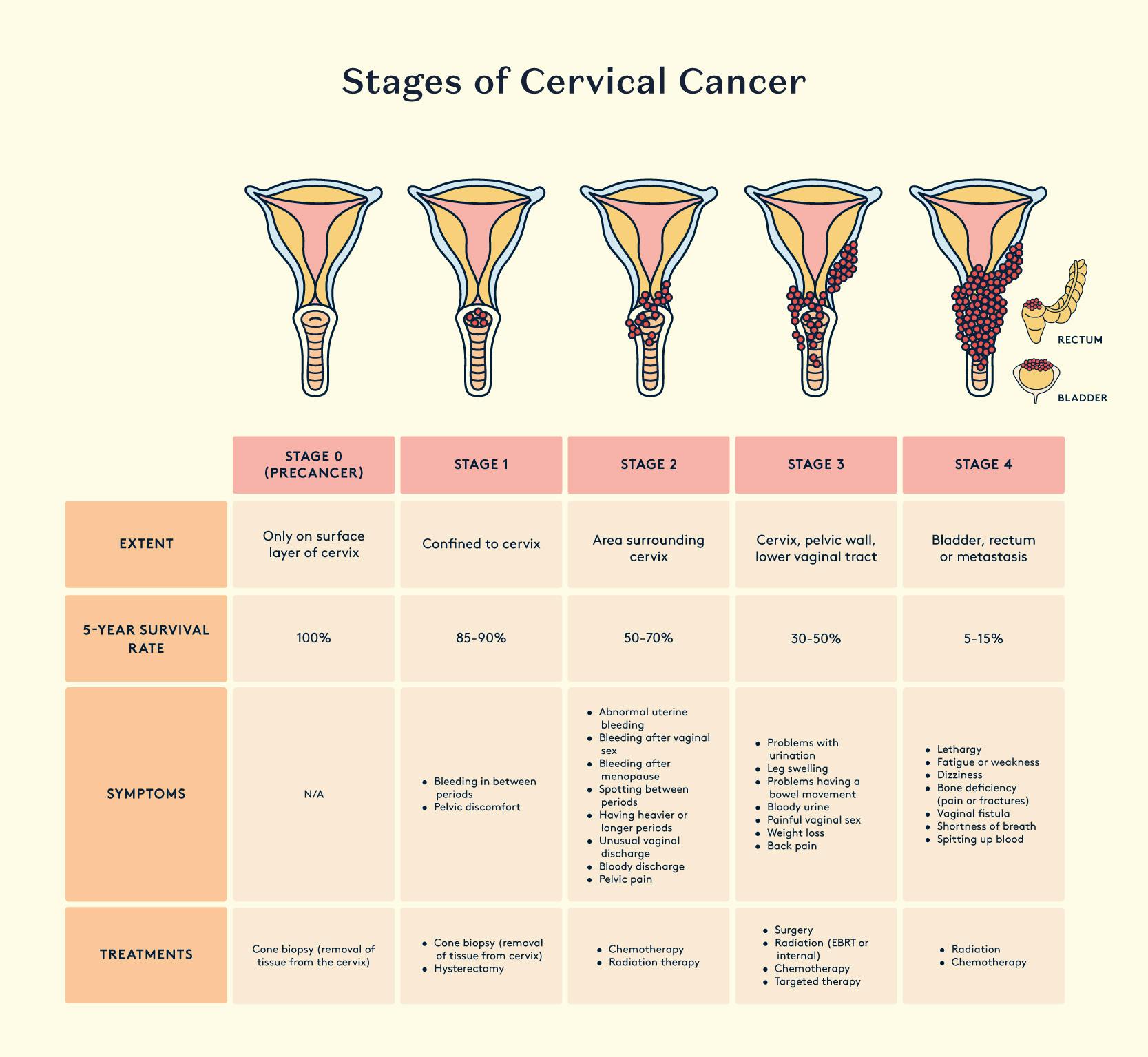
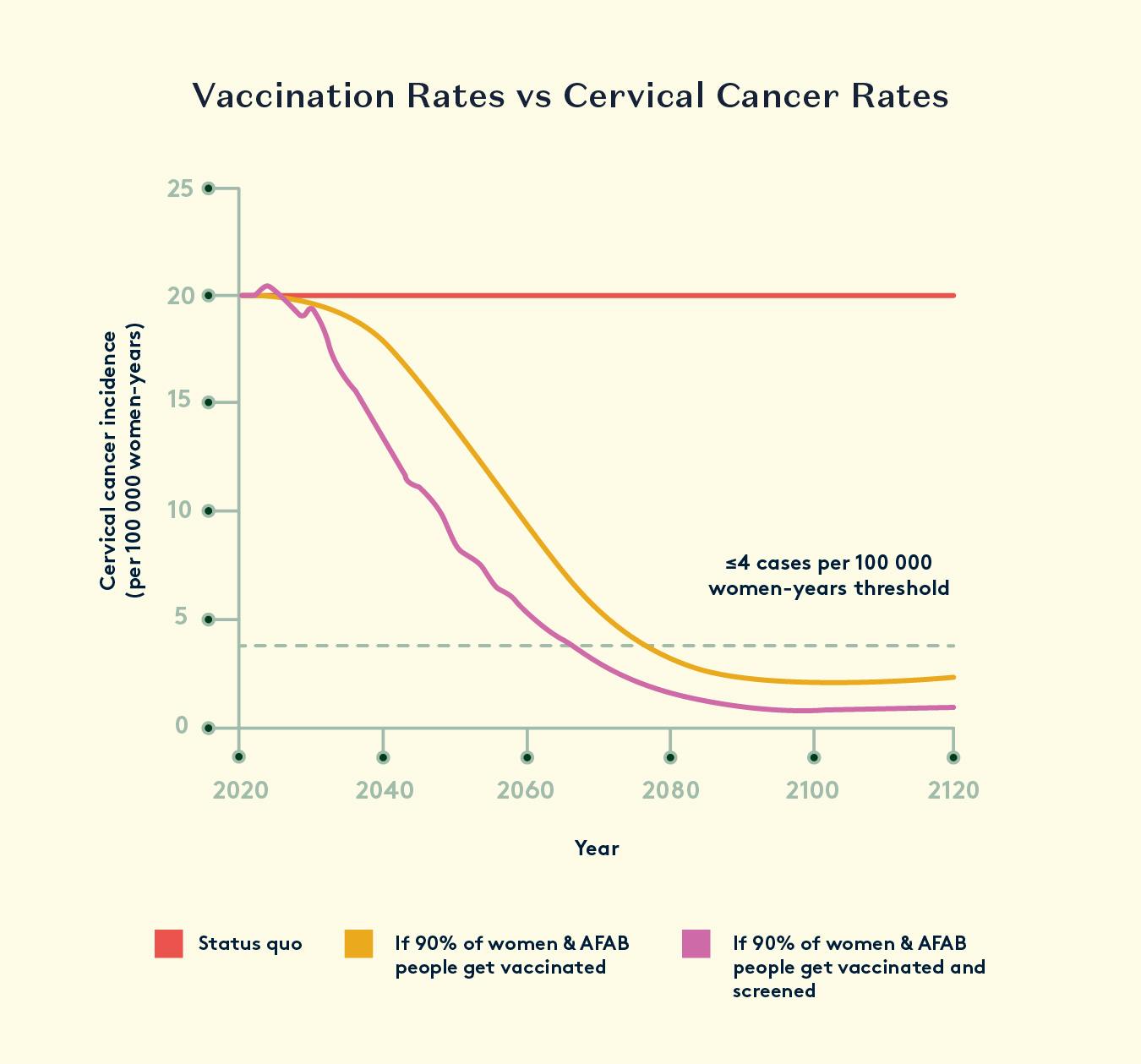
While HPV vaccination prevents infection and subsequent disease, the secondary prevention of cervical cancer can be achieved through the early detection of HPV infection and cervical abnormalities by cervical screening. The introduction of a national cervical screening programme in the UK with coordinated call and recall was responsible for a major fall in the incidence and death rate from cervical cancer. It has been estimated that mortality rates fell approximately 60% between 1974 and 2004 in the UK due to cervical screening.
Today, high-risk HPV infections are fully preventable, thanks to the successful vaccination programs available in most countries. This, in combination with certain lifestyle choices are the path towards eradication of cervical cancer.
Oral cancer
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer globally. It can vary in occurrence, with Europe reporting 3–5% of cancers as oral and oropharyngeal, while in parts of Southeast Asia and India, it's as high as 40–50%,. Smoking, betel nut chewing, and alcohol abuse contribute to most HNSCC cases.
HPV has been found in 45–95% of the mouth and throat cancers,. While the overall incidence of head and neck squamous cell carcinoma is decreasing due to reduced smoking rates, there's a rise in oropharyngeal SCC cases,,. Human papillomavirus (HPV) is implicated in oropharyngeal cancer development, recognized by the Agency for Research on Cancer (IARC) as a risk factor. Molecular and epidemiological data indicate that high-risk HPV types cause a subset of oropharyngeal cancer. In the Western world, HPV-positive cases now dominate, showing better outcomes than HPV-negative patients. However, the natural history and biology of HPV infection in head and neck tumours are not fully understood.
The prognosis for HPV-related head and neck cancer is better than that for non-HPV cancers. Studies have shown 80-95% survival rate in 2-3 years, compared to 57-62% for the non-HPV group,.
The known risk-factors for HPV-related head and neck cancer include:
- Increasing number of vaginal and oral sex partners
- History of genital warts and marijuana use
- Tobacco and alcohol use
Symptoms vary, based on the specific area affected.
Oral cavity:
- white or red patch on the gums, tongue, or the inside of the mouth
- growth or swelling in the jaw that makes dentures uncomfortable,
- unusual bleeding or pain in the mouth.
Throat (Pharynx):
- pain while swallowing
- persistent neck or throat pain
- ringing or pain in the ears, or difficulty hearing.
Voice box (Larynx):
- Difficulty breathing or speaking,
- Pain during swallowing,
- Ear pain.
Paranasal sinuses and nasal cavity:
- Blocked sinuses that don't clear
- Chronic sinus infections that don't respond to antibiotics
- Nosebleeds
- Frequent headaches
- Swelling or other issues with the eyes
- Pain in upper teeth
- Problems with dentures.
Salivary Glands:
- swelling under chin or around jawbone
- numbness or paralysis of facial muscles
- or persistent pain in face, chin, or neck
Penile cancer
Penile squamous cell carcinoma (PSCC) is a rare cancer, especially in high-income countries, where it's found in about 0.1–1 in 100,000 men. However, in certain parts of Africa, Asia, and South America, it can make up to 10% of cancers in men. Things that increase the risk of PSCC include not being circumcised as a child, a condition called phimosis, ongoing inflammation, not keeping the penis clean, smoking, a weak immune system, and being infected with human papillomavirus (HPV). There are different types of PSCC, some related to HPV and some not, each having different outlooks.
If the cancer is only in one area, it can often be treated effectively with creams, surgery, or radiation. In more advanced cases, a mix of treatments is needed, but the best order and which patients benefit most from each treatment are still being studied.
Both localised and advanced penile cancers, along with their treatments, can significantly impact the lives of patients. They can affect sexual and urinary function and lead to swelling, which can have a big impact on a person's quality of life.
A review of 1,266 cases of penile cancer, revealed an overall HPV prevalence of 47.9%, where the most common HPV types identified were HPV16, and HPV18, present in 36.7% of cases.
Anal cancer
Every year, around 27,000 new cases of anal cancer are reported globally, and the female-to-male ratio can be as high as 5:1. Recent studies indicate that rates of anal cancers in females have more than doubled across all races and ages. Although white women have higher rates than black women, the reverse is true for males, with black men having significantly higher rates of anal squamous cell carcinomas.
Studies in Sweden and Denmark found that 83% to 95% of anal cancer patients were positive for HPV. In the US, anal cancer incidence rates were notably higher in people coinfected with HIV, especially in men who have sex with men (MSM), older individuals, and those with AIDS.
Risk factors for anal cancer:
- Being a man who has sex with men (MSM)
- HIV infection
- Solid organ transplant recipients
- History of venereal diseases
- Smoking
- Crohn's disease
Symptoms of anal cancer:
- Rectal bleeding or blood in the stools
- Small lumps seen or felt around the anus which could be confused with piles
- An increase in the number or size of piles
- Pain in the anal area – affects about 30% of people
- Difficulty in passing stools and extreme constipation are common symptoms
- Feeling a continuous urge to pass a motion, with no production, possibly with increased mucus
- Discharge from the back passage, or swelling, itching and persistent redness or soreness around the anal area
- Difficulty controlling your bowels (faecal incontinence)
- One or more lumps in the groin area.
Reproductive implications
HPV infection in semen poses a risk factor for male infertility, as the virus binds to segments of the sperm head or tail, compromising its functionality,. Studies also suggest that HPV in sperm may increase the risk of assisted reproductive technology (ART) failure, although more evidence is needed to confirm this. While there is substantial research on the association between male infertility and HPV, the impact of HPV on fertility or pregnancy outcomes in women has been less explored.
Women facing infertility issues are twice as likely to exhibit HPV-related abnormal cervical cytology or high-grade cervical lesions compared to the general population. Furthermore, procedures like removing cancerous or precancerous cells from the cervix can impact a woman's ability to carry a pregnancy to full-term.
HPV during pregnancy
During pregnancy, the immune system of the mother adapts to maintain tolerance towards the foetus. It suspected that this is why certain low-risk strains, such as HPV types 6 and 11, which cause genital warts that may increase in size and number during pregnancy. While these are benign, enlarged genital warts in pregnant women can make vaginal delivery more difficult and lead to the occurrence of recurrent respiratory papillomatosis in the infant. Studies and systematic reviews suggest that HPV infections may be more common during pregnancy, likely explained by their increased persistence due to the adapted responses of the immune system. However, in the postpartum period, as the organism recovers, an increase in HPV clearance is observed.
Interestingly, HPV DNA has been found in the placenta, amniotic fluid, and umbilical cord, which explains the observed vertical transmission to the infant,,.
The role of HPV infection in adverse pregnancy outcomes is not fully clear, as existing evidence is contradicting. Some studies show no relationships, while others discovered a link to preterm birth, spontaneous abortion, pregnancy-induced hypertensive disorders to intrauterine growth restriction and low birth weight.
Diagnosis & tests
HPV testing is used as a tool for cervical cancer screening alone, despite its well-established implication in other cancers.
The diagnosis of human papillomavirus (HPV) has evolved significantly over the years, with various methods being developed to detect and identify the virus. Prior to the 1990s, the clinical utility of HPV screening was not widely recognized, and other tests, such as the well-known Pap Smear (invented in 1928), were used to detect cervical cancer through cytology (testing for cell changes).
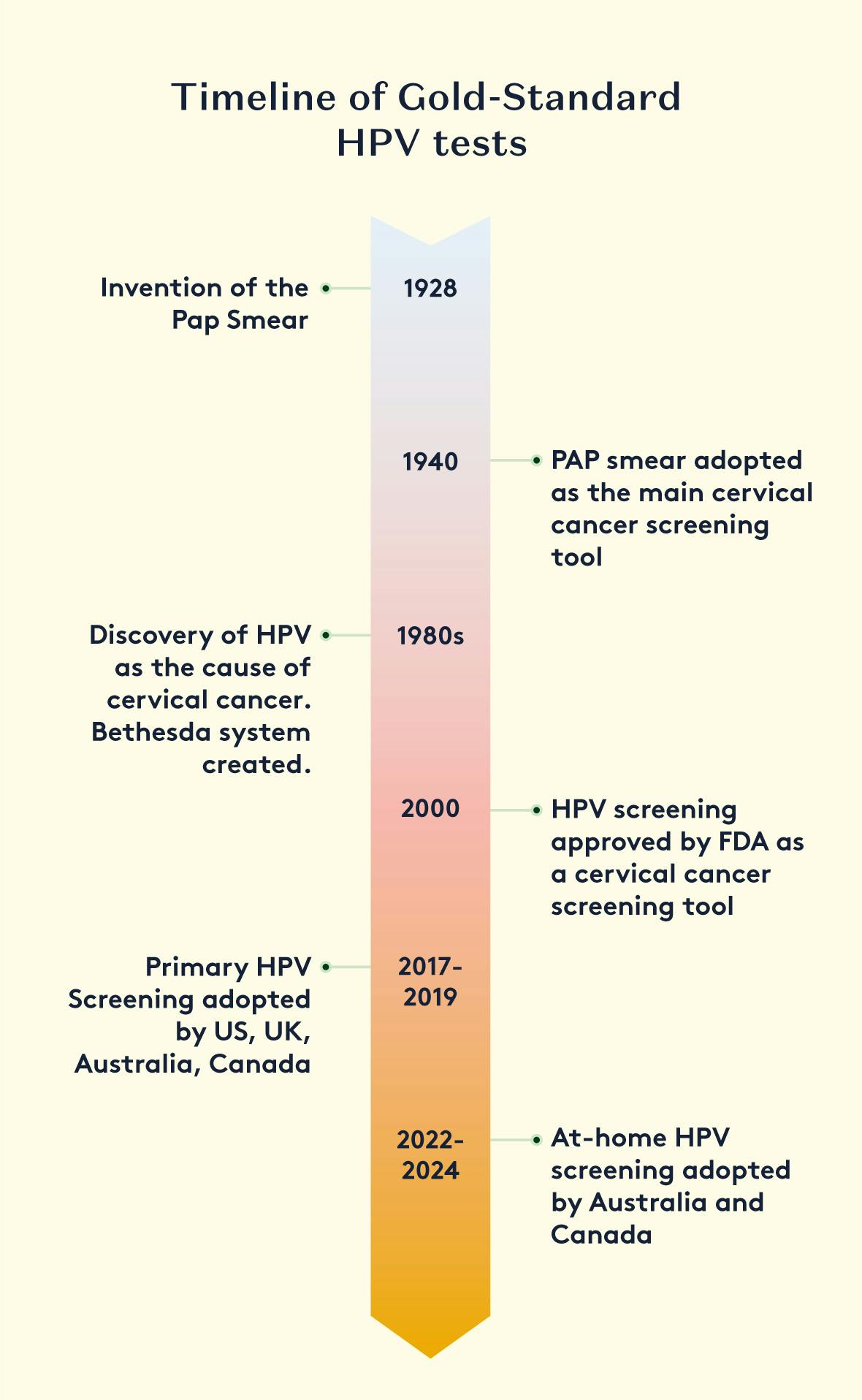
In the 1980s, HPV was recognized as the primary cause of cervical cancer. This led to the creation of the Bethesda system, a standardised framework for reporting cervical cytology results that incorporated lab results for high-risk HPV strains. Screening programs were then updated to include both cytology and HPV testing on Pap smear samples, with the results reported together
HPV was recognised as the cause of cervical cancer in the 1980s. A new system was created for reporting cytology results, which incorporated lab results for high-risk HPV strains, called the Bethesda system. Screening programmes were changed, so that samples from a Pap Smear underwent both cytology and HPV testing. Results were reported together.
At the start of the 21st century, HPV screening was established as a standalone cervical cancer screening tool. Data showed that a positive HPV result, followed by cytology, was a better predictor of cervical cancer compared to cytology alone. This set the foundation for Primary HPV Screening, which is now the preferred approach in many regions.
In Primary HPV Screening, a vaginal or cervical sample is first tested for the presence of high-risk HPV strains. Only samples that test positive for HPV are then also tested for cytological abnormalities. Samples that test negative for HPV do not require further testing, and patients are recalled for screening after 1, 3, or 5 years, depending on the national screening guidelines.
There are various methods for detecting HPV from a human sample, ranging in complexity and diagnostic accuracy. Today, PCR (Polymerase Chain Reaction) is recognised as the gold-standard and most sensitive method for detecting HPV with high accuracy and efficiency.
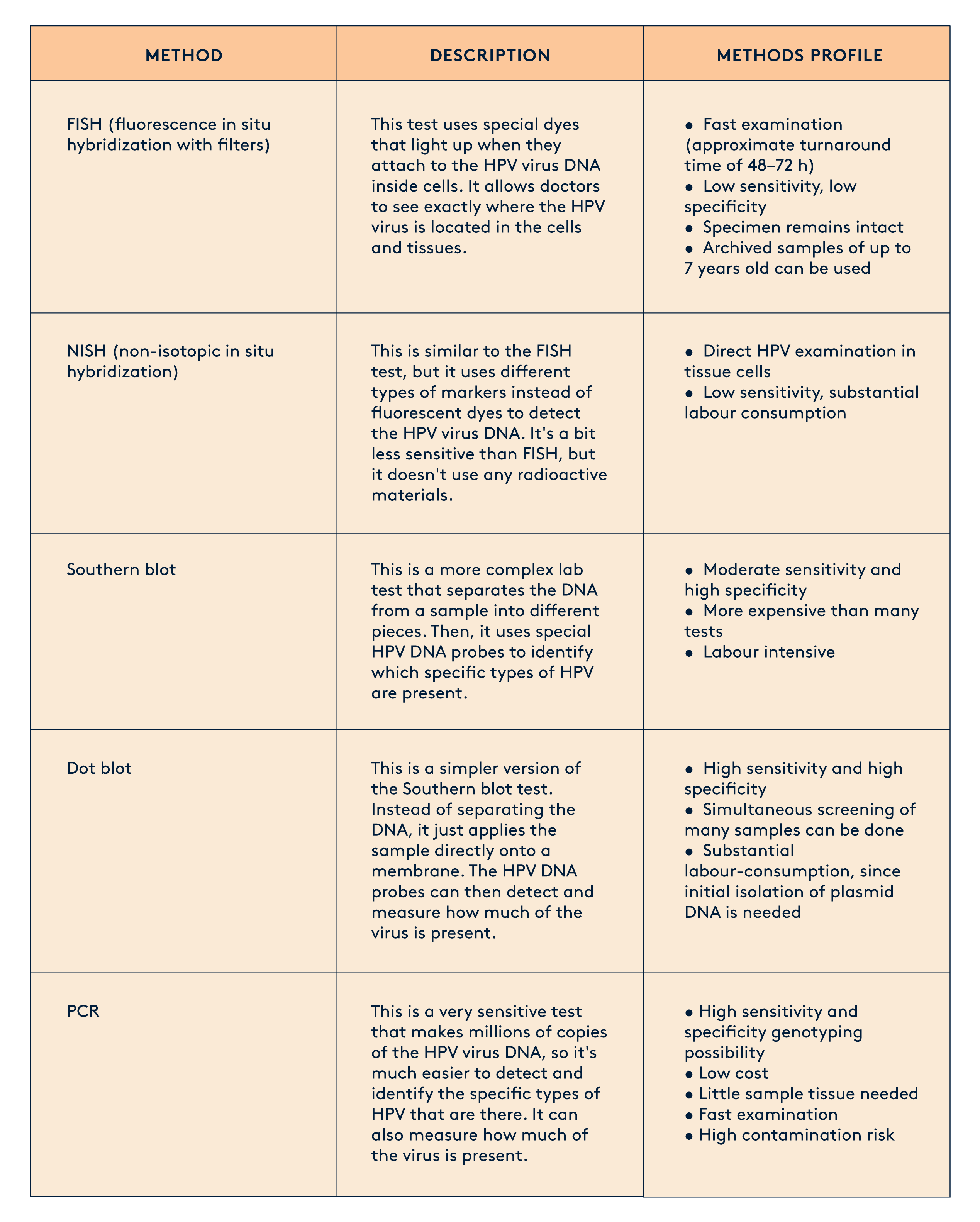
Sample Collection Devices and Methods for HPV Screening
The diagnosis and screening of human papillomavirus (HPV) relies on the collection of appropriate samples from the patient. Over the years, various sample collection devices and methods have been developed to facilitate HPV testing.
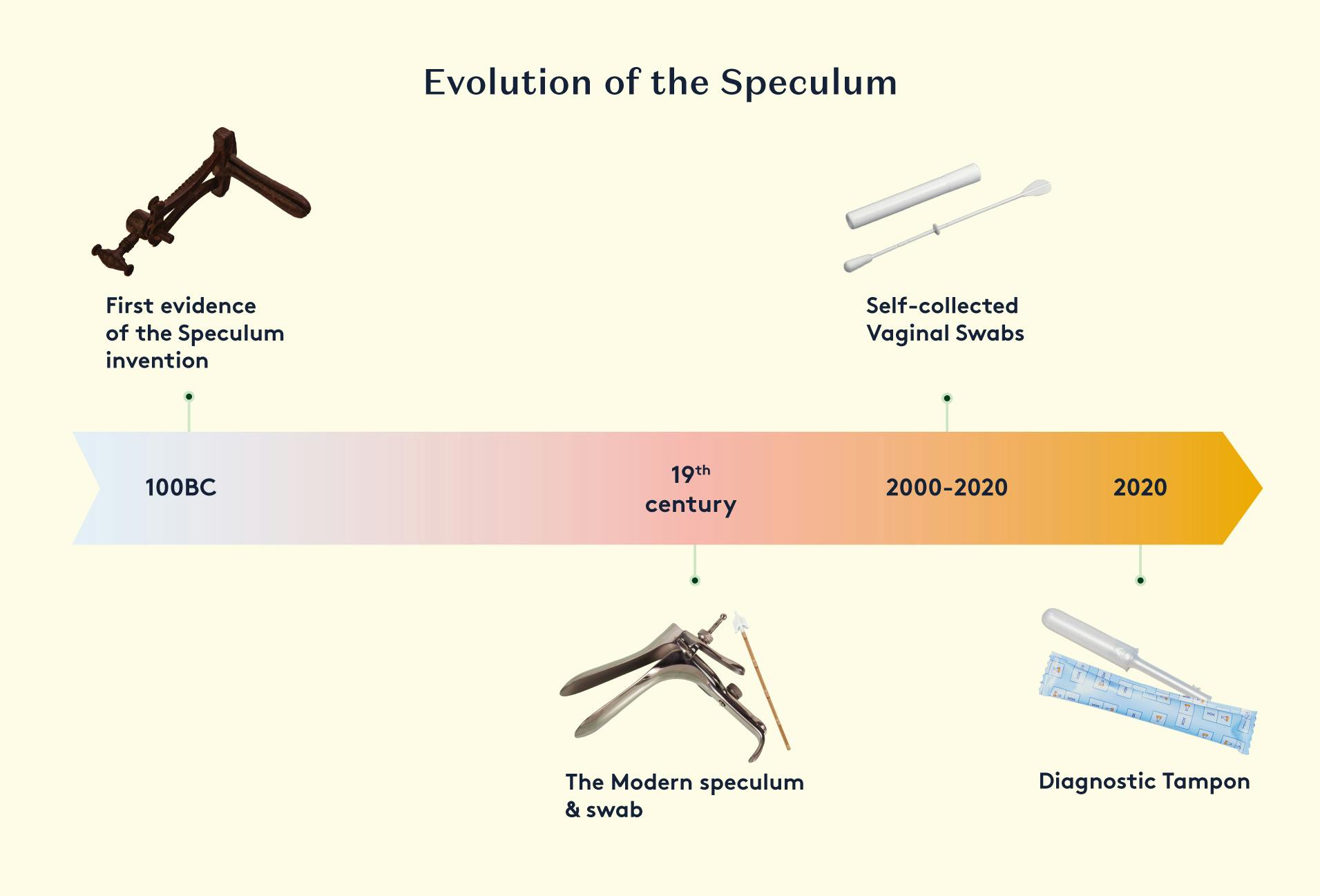
Clinician-Collected Samples
Traditionally, HPV testing has been performed on samples collected by a healthcare provider during a pelvic examination. This typically involves the use of a specialised brush or swab to obtain a sample of cervical cells.
The healthcare provider inserts a speculum into the vagina to visualise the cervix, and then uses the brush or swab to gently collect cells from the surface of the cervix. The sample is then placed in a collection tube containing a preservative solution.
Several studies indicate that many women and AFAB individuals consider the speculum used during Pap smear testing to be invasive and uncomfortable, which can lead them to skip or avoid cervical cancer screening. The main reasons cited include fear and embarrassment, as well as pain and discomfort, caused by the speculum,.
Self-Collected Samples
In recent years, there has been a growing focus on enabling patients to collect their own samples for HPV testing. This self-collection approach can improve access to screening, especially in underserved populations.
Some of the self-collection devices that have been developed and studied include brushes, swabs and tampon-based devices. The collected samples can be stored in a preservative solution and sent to a laboratory for HPV testing.
Prevention and vaccination
In 2018 the World Health Organization announced its aim to eliminate cervical cancer and made a global call for action. Success with this would result in the disappearance of a cancer with a global incidence of around 530,000 cases per year, approximately half of whom will die of the disease. Prevention of all human papillomavirus (HPV)-related cancers would also remove another 80,000 cancers of anogenital and oropharyngeal origin globally, accounting for about 5% of all cancers. This is a small but important contribution to eliminating cancer, especially for the low- and middle-income countries where there is no effective screening for cervical cancer.
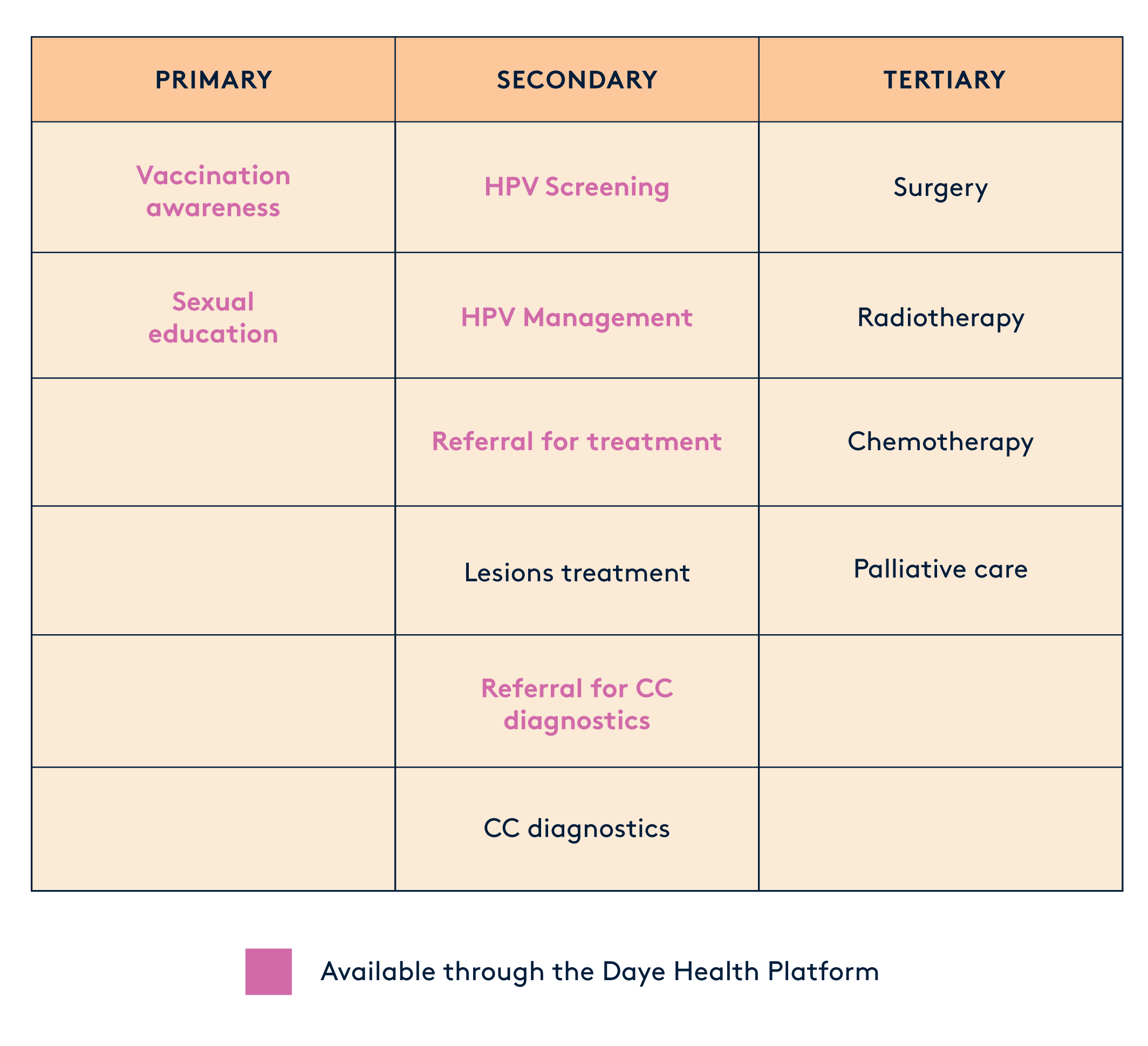
Prevention strategies against HPV-related disease are divided into 3 categories:
Primary Prevention. This includes strategies to stop the problem before it starts. It includes getting vaccinated against HPV, which is the virus that can lead to cervical cancer and education programmes about safe sex practices.
Secondary Prevention. Here, we're talking about catching the issue early. This involves screening for and treating any precancerous lesions before they become a big problem.
Tertiary Prevention. If cancer has already developed, this step focuses on diagnosing and treating it to prevent it from spreading further.
The HPV Vaccine
The HPV vaccines are effective at protecting against HPV infection related diseases; however, they are prophylactic and cannot be used as treatment of already existing infections.
For long-lasting protection against HPV throughout our sexually active years, vaccines need to induce high and sustained levels of antibodies. The ideal HPV vaccine should offer improved protection against all high-risk HPV types.
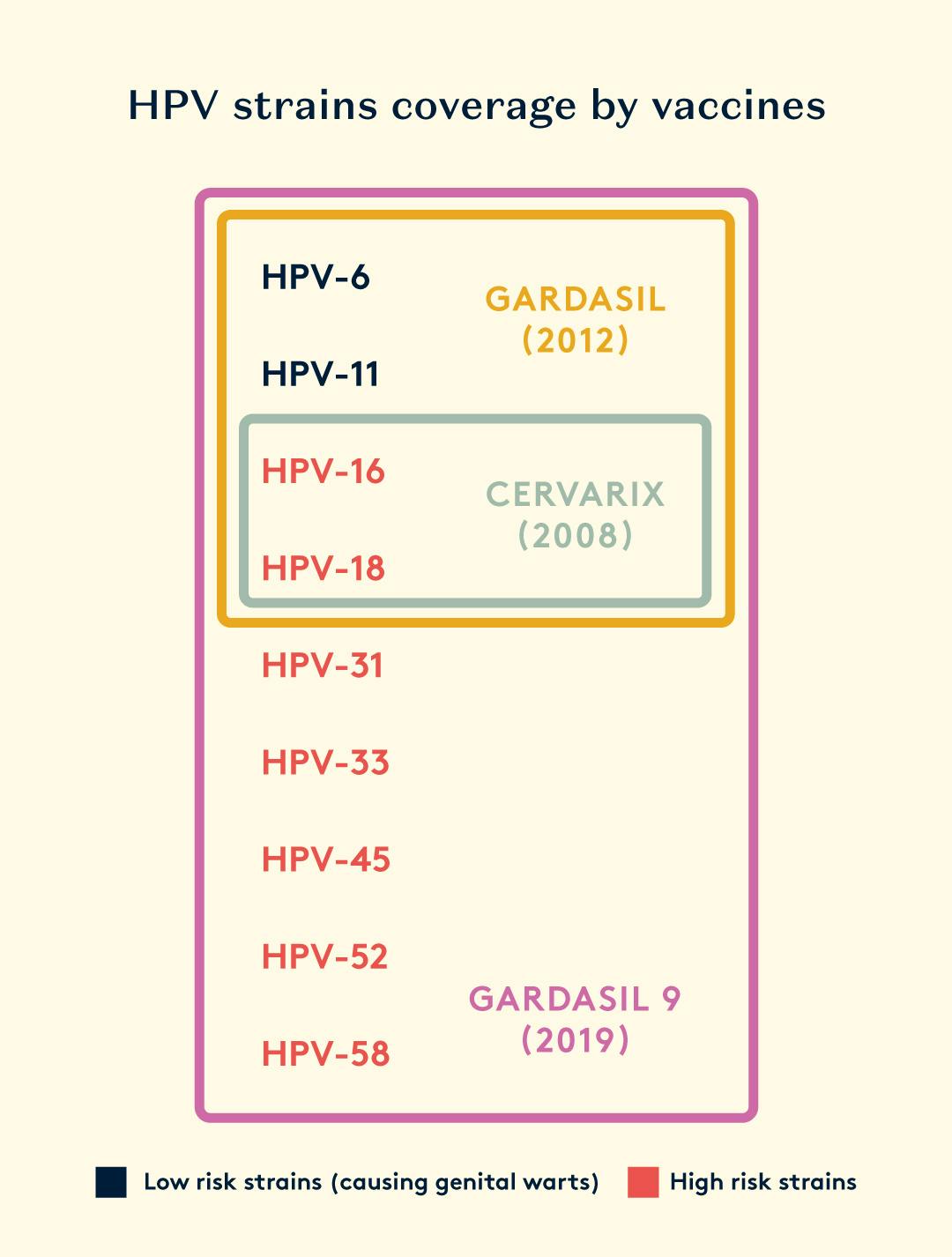
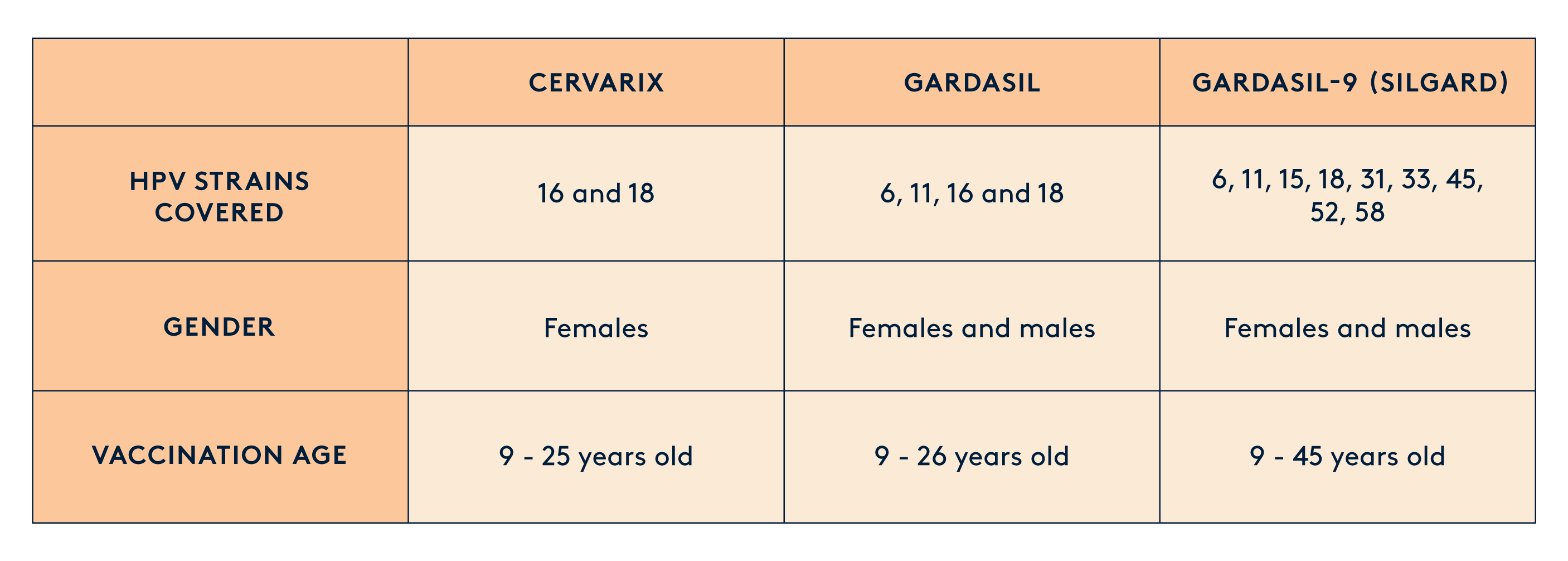
There are three preventive vaccines, which protect against HPV infections by inducing high and long-lasting levels of antibodies. However, they have limitations in eliminating existing infections and offering broad protection. These vaccines cover a different combination of HPV strains - all of which have shown to be 70-90% effective in preventing HPV-related cancers. They've also been proven to be safe in various studies and real-world use. The quadrivalent and nonavalent vaccines even protect against the types of HPV that cause anogenital warts.
To date, the HPV vaccination scheme approved by the Food and Drug Administration in the US and MHRA in the UK includes three recombinant vaccines: bivalent (Cervarix–types 16 and 18), quadrivalent (Gardasil–types 6, 11, 16, 18), and nonavalent (Gardasil-9 types 6, 11, 16, 18, 31, 33, 45, 52, and 58).
Gardasil and Gardasil 9 vaccines are usually given in three doses, spaced out at 0, 2, and 6 months. But, for girls under 15, they can now get only two shots at 0 and 6 months. Cervarix also needs three shots at 0, 1, and 6 months.
By getting vaccinated against HPV and regularly screening for precancerous and cancerous lesions, we can significantly lower the chances of developing HPV-related diseases and reduce the risk of death.
Where can I get the vaccine from?
If you are a student, check with your school or college health services. Many educational institutions host vaccination services to ensure students are protected against preventable diseases like HPV. You can also reach out directly to public Vaccination Services in your country. In the UK, this is the NHS Community Trust.
Your primary care doctor, gynaecologist, or local health clinic are common places to receive the HPV vaccine. You may qualify for a free vaccine, but eligibility varies from country to country. In the UK, for example, you can get vaccinated for free if you are:
- A woman or AFAB individual under the age of 25
- A boy born after September, 2006 and under the age of 25
- A sex worker under the age of 45
- A transexual woman under the age of 45
- A nan who has sex with men under the age of 45
You can schedule an appointment with your healthcare provider to discuss vaccination, and they can guide you through the process.
If you are unable to get the vaccine for free through a healthcare provider or your school, you can also buy it privately. Some pharmacies and drugstores provide HPV vaccinations. Call your local pharmacy to inquire about the availability of the HPV vaccine and whether you need an appointment.
Debunking Myths about HPV
Myth: HPV is not harmful to men.
Fact: HPV can infect both men and women. While certain strains can lead to cervical cancer in women, men can also develop genital warts and are at risk of HPV-related cancers in the genitals and throat.
Myth: Only sexually active individuals can get HPV.
Fact: HPV is primarily a sexually transmitted infection, but it can also be spread through intimate skin-to-skin contact. It's possible to contract the virus even if one has had limited sexual partners or is not currently sexually active.
Myth: HPV always leads to noticeable symptoms.
Fact: Many people with HPV may not show any symptoms. Consequently, individuals may unknowingly transmit the virus to others. Regular screenings, including Pap smears and HPV tests, are crucial for early detection of HPV-related issues.
Myth: Condoms provide complete protection against HPV.
Fact: While condoms can reduce the risk of HPV transmission, they do not provide complete protection. This is because the virus can infect areas not covered by the condom. Vaccination remains a more effective preventive measure.
Myth: HPV is only a concern for young people.
Fact: HPV infections and related diseases can occur at any age. While the risk is higher for those who are sexually active, including young adults, individuals of all ages can contract the virus. Vaccination is recommended for adolescents and young adults, but older individuals can still benefit.
Myth: If you've had HPV and it clears up, you can't get infected again.
Fact: While the immune system can clear many HPV infections, it's still possible to get infected with different HPV strains. Previous infection does not necessarily confer immunity to all HPV types.
Myth: HPV vaccines are only for women.
Fact: HPV vaccines are recommended for both men and women. Vaccination not only protects against cervical cancer in women but also helps prevent genital warts and various cancers in men. It contributes to herd immunity, reducing the overall prevalence of the virus.
Myth: Getting the HPV vaccine means you don't need regular screenings.
Fact: Vaccination is a preventive measure, but it does not replace the need for regular screenings. Screenings like Pap smears and HPV tests are essential for early detection and treatment of potential HPV-related issues.
Myth: The HPV vaccine is not safe and may cause serious side effects.
Fact: Extensive research and clinical trials have consistently demonstrated the safety and efficacy of HPV vaccines. Serious side effects are extremely rare, and the benefits of vaccination in preventing HPV-related cancers and diseases far outweigh any potential risks. The vaccine has undergone rigorous testing and is continually monitored for safety. Like any vaccine, some individuals may experience mild side effects, such as redness or soreness at the injection site, but severe adverse reactions are exceptionally uncommon.
For any cancer-related questions or if you need support—emotional, practical, physical, or financial—reach out to Macmillan Cancer Support. They're there for you. Another fantastic resource is The Eve Appeal, the UK's top charity for research and awareness on the five gynaecological cancers: womb, ovarian, cervical, vulvar, and vaginal. They also provide a nurse-led information service to answer any questions you might have about gynaecological health.
Resources
- Lopez-Bueno A, Mavian C, Labella AM, Castro D, Borrego JJ, Alcami A, et al.. Concurrence of iridovirus, polyomavirus, and a unique member of a new group of fish papillomaviruses in lymphocystis disease-affected gilthead sea bream. J Virol. (2016) 90:8768–79. 10.1128/JVI.01369-16
- http://pave.niaid.nih.gov/ (accessed on 16 February 2021)
- Diane M. Harper, Leslie R. DeMars, HPV vaccines – A review of the first decade, Gynecologic Oncology, Volume 146, Issue 1, 2017, Pages 196-204, https://doi.org/10.1016/j.ygyno.2017.04.004.
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; de Sanjose, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Prim. 2016, 2, 16086.
- McBride, A.A. Mechanisms and strategies of papillomavirus replication. Biol. Chem. 2017, 398, 919–927.
- Hammer, A.; de Koning, M.N.; Blaakaer, J.; Steiniche, T.; Doorbar, J.; Griffin, H.; Mejlgaard, E.; Svanholm, H.; Quint, W.G.; Gravitt, P.E. Whole tissue cervical mapping of HPV infection: Molecular evidence for focal latent HPV infection in humans. Papillomavirus Res. 2019, 7, 82–87.
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C.; Murakami, I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 2015, 25 (Suppl. 1), 2–23.
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; de Sanjose, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Prim. 2016, 2, 16086.
- Antonsson A, Forslund O, Ekberg H, Sterner G, Hansson BG. The ubiquity and impressive genomic diversity of human skin papillomaviruses suggest a commensalic nature of these viruses. J Virol. (2000) 74:11636–41. 10.1128/JVI.74.24.11636-11641.2000
- Plasmeijer, E.I.; Struijk, L.; Bouwes Bavinck, J.N.; Feltkamp, M.C. Epidemiology of cutaneous human papillomavirus infections. Cancer Treat. Res. 2009, 146, 143–157.
- Gheit T. Mucosal and Cutaneous Human Papillomavirus Infections and Cancer Biology. Front Oncol. 2019 May 8;9:355. doi: 10.3389/fonc.2019.00355. PMID: 31134154; PMCID: PMC6517478.
- Stanley, M. Immune Responses to Human Papillomavirus and the Development of Human Papillomavirus Vaccines. In Human Papillomavirus; Elsevier: Amsterdam, The Netherlands, 2020; pp. 283–297.
- Jang, M.K.; Shen, K.; McBride, A.A. Papillomavirus genomes associate with BRD4 to replicate at fragile sites in the host genome. PLoS Pathog. 2014, 10, e1004117.
- Mitra A, MacIntyre DA, Ntritsos G, Smith A, Tsilidis KK, Marchesi JR, et al. The Vaginal Microbiota Associates With the Regression of Untreated Cervical Intraepithelial Neoplasia 2 Lesions. Nat Commun (2020) 11 (1):1999. doi: 10.1038/s41467-020-15856-y
Usyk M, Zolnik CP, Castle PE, Porras C, Herrero R, Gradissimo A, et al. Cervicovaginal Microbiome and Natural History of HPV in a Longitudinal Study. PloS Pathog (2020) 16(3):e1008376. doi: 10.1371/journal. Ppat.1008376
Berggrund M, Gustavsson I, Aarnio R, Lindberg JH, Sanner K, Wikstrom I, et al. Temporal Changes in the Vaginal Microbiota in Self-Samples and its Association With Persistent HPV16 Infection and CIN2. Virol J (2020) 17 (1):147. doi: 10.1186/s12985-020-01420-z
Brotman RM, Shardell MD, Gajer P, Tracy JK, Zenilman JM, Ravel J, et al. Interplay Between the Temporal Dynamics of the Vaginal Microbiota and Human Papillomavirus Detection. J Infect Dis (2014) 210(11):1723��–33. doi: 10.1093/infdis/jiu330
Di Paola M, Sani C, Clemente AM, Iossa A, Perissi E, Castronovo G, et al. Characterization of Cervico-Vaginal Microbiota in Women Developing Persistent High-Risk Human Papillomavirus Infection. Sci Rep (2017) 7 (1):10200. doi: 10.1038/s41598-017-09842-6 - Kovachev S. Defence Factors of Vaginal Lactobacilli. Crit Rev Microbiol (2018) 44(1):31–9. doi: 10.1080/1040841X.2017.1306688
- Cheng L, Norenhag J, Hu YOO, Brusselaers N, Fransson E, Ahrlund-Richter A, et al. Vaginal Microbiota and Human Papillomavirus Infection Among Young Swedish Women. NPJ Biofilms Microbiomes (2020) 6(1):39. doi: 10.1038/s41522-020-00146-8
Zhou Y, Wang L, Pei F, Ji M, Zhang F, Sun Y, et al. Patients With Lr-Hpv Infection Have a Distinct Vaginal Microbiota in Comparison With Healthy Controls. Front Cell Infect Microbiol (2019) 9:294. doi: 10.3389/fcimb. 2019.00294
Oh HY, Kim BS, Seo SS, Kong JS, Lee JK, Park SY, et al. The Association of Uterine Cervical Microbiota With an Increased Risk for Cervical Intraepithelial Neoplasia in Korea. Clin Microbiol Infect (2015) 21(7):674 e1–9. doi: 10.1016/j.cmi.2015.02.026
Mitra A, MacIntyre DA, Lee YS, Smith A, Marchesi JR, Lehne B, et al. Cervical Intraepithelial Neoplasia Disease Progression Is Associated With Increased Vaginal Microbiome Diversity. Sci Rep (2015) 5:16865. doi: 10.1038/srep16865 - M. L. Gillison, “Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity,” Seminars in Oncology, vol. 31, no. 6, pp. 744–754, 2004.
- F. Bray, R. Sankila, J. Ferlay, and D. M. Parkin, “Estimates of cancer incidence and mortality in Europe in 1995,” European Journal of Cancer, vol. 38, no. 1, pp. 99–166, 2002.
- J. J. Pindborg, K. H. Zheng, C. R. Kong, and F. X. Lin, “Pilot survey of oral mucosa in areca (betel) nut chewers on Hainan Island of the People's Republic of China,” Community Dentistry and Oral Epidemiology, vol. 12, no. 3, pp. 195–196, 1984.
- L. Licitra, J. Bernier, C. Grandi, M. Merlano, P. Bruzzi, and J. L. Lefebvre, “Cancer of the oropharynx,” Critical Reviews in Oncology/Hematology, vol. 41, no. 1, pp. 107–122, 2002.
- A. Näsman, P. Attner, L. Hammarstedt et al., “Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma?” International Journal of Cancer, vol. 125, no. 2, pp. 362–366, 2009.
- L. Hammarstedt, D. Lindquist, H. Dahlstrand et al., “Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer,” International Journal of Cancer, vol. 119, no. 11, pp. 2620–2623, 2006.
- E. M. Sturgis and P. M. Cinciripini, “Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers?” Cancer, vol. 110, no. 7, pp. 1429–1435, 2007.
- M. Frisch, H. Hjalgrim, A. B. Jæger, and R. J. Biggar, “Changing patterns of tonsillar squamous cell carcinoma in the United States,” Cancer Causes and Control, vol. 11, no. 6, pp. 489–495, 2000.
- I. U. Doobaree, S. H. Landis, K. M. Linklater, I. El-Hariry, H. Moller, and J. Tyczynski, “Head and neck cancer in South East England between 1995–1999 and 2000–2004: an estimation of incidence and distribution by site, stage and histological type,” Oral Oncology, vol. 45, no. 9, pp. 809–814, 2009.
- G. D'Souza, A. R. Kreimer, R. Viscidi et al., “Case-control study of human papillomavirus and oropharyngeal cancer,” New England Journal of Medicine, vol. 356, no. 19, pp. 1944–1956, 2007.
- C. Fakhry, W. H. Westra, S. Li et al., “Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial,” Journal of the National Cancer Institute, vol. 100, no. 4, pp. 261–269, 2008.
- P. Attner, J. Du, A. Näsman et al., “The role of human papillomavirus in the increased incidence of base of tongue cancer,” International Journal of Cancer, vol. 126, no. 12, pp. 2879–2884, 2010.
- Thomas, A., Necchi, A., Muneer, A. et al. Penile cancer. Nat Rev Dis Primers 7, 11 (2021). https://doi.org/10.1038/s41572-021-00246-5
- Backes, D.M., Kurman, R.J., Pimenta, J.M. et al. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control 20, 449–457 (2009). https://doi.org/10.1007/s10552-008-9276-9
- Bertolotti, A.; Milpied, B.; Fouéré, S.; Dupin, N.; Cabié, A.; Derancourt, C. Local Management of Anogenital Warts in Non-immunocompromised Adults: A Systematic Review and Meta-analyses of Randomized Controlled Trials. Dermatol. Ther. 2019, 9, 761–774.
- Rombaldi, R.L.; Serafini, E.P.; Mandelli, J.; Zimmermann, E.; Losquiavo, K.P. Perinatal transmission of human papilomavirus DNA. Virol. J. 2009, 6, 83. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Xu, L.; Sun, Y.; Wang, Z. The prevalence and risk of human papillomavirus infection in pregnant women. Epidemiol. Infect. 2014, 142, 1567–1578.
- Nobbenhuis, M.A.E.; Helmerhorst, T.J.M.; van den Brule, A.J.C.; Rozendaal, L.; Bezemer, P.D.; Voorhorst, F.J.; Meijer, C.J.L.M. High-risk human papillomavirus clearance in pregnant women: Trends for lower clearance during pregnancy with a catch-up postpartum. Br. J. Cancer 2002, 87, 75–80
- Gomez, L.M.; Ma, Y.; Ho, C.; McGrath, C.M.; Nelson, D.B.; Parry, S. Placental infection with human papillomavirus is associated with spontaneous preterm delivery. Hum. Reprod. 2008, 23, 709–715.
- Armbruster-Moraes, E.; Ioshimoto, L.M.; Leao, E.; Zugaib, M. Detection of human papillomavirus deoxyribonucleic acid sequences in amniotic fluid during different periods of pregnancy. Am. J. Obstet. Gynecol. 1993, 169, 1074.
- Koskimaa, H.M.; Waterboer, T.; Pawlita, M.; Grénman, S.; Syrjänen, K.; Syrjänen, S. Human papillomavirus genotypes present in the oral mucosa of newborns and their concordance with maternal cervical human papillomavirus genotypes. J. Pediatr. 2012, 160, 837–843.
- Aldhous, M.C.; Bhatia, R.; Pollock, R.; Vragkos, D.; Cuschieri, K.; Cubie, H.A.; Norman, J.E.; Stock, S.J. HPV infection and pre-term birth: A data-linkage study using Scottish Health Data. Wellcome Open Res. 2019, 4, 48.
- Xiong, Y.Q.; Mo, Y.; Luo, Q.M.; Huo, S.T.; He, W.Q.; Chen, Q. The Risk of Human Papillomavirus Infection for Spontaneous Abortion, Spontaneous Preterm Birth, and Pregnancy Rate of Assisted Reproductive Technologies: A Systematic Review and Meta-Analysis. Gynecol. Obstet. Investig. 2018, 83, 417–427
- Niyibizi, J.; Zanré, N.; Mayrand, M.-H.; Trottier, H. The association between adverse pregnancy outcomes and maternal human papillomavirus infection: A systematic review protocol. Syst. Rev. 2017, 6, 53.
- Ford, J.H.; Li, M.; Scheil, W.; Roder, D. Human papillomavirus infection and intrauterine growth restriction: A data-linkage study. J. Matern.-Fetal Neonatal Med. 2019, 32, 279–285.
- Niyibizi, J.; Zanré, N.; Mayrand, M.-H.; Trottier, H. Association Between Maternal Human Papillomavirus Infection and Adverse Pregnancy Outcomes: Systematic Review and Meta-Analysis. J. Infect. Dis. 2020, 221, 1925–1937.
- Mark W. Tomlinson, Thomas J. Caruthers, Janice E. Whitty, Bernard Gonik, Does delivery improve maternal condition in the respiratory-compromised gravida?, Obstetrics & Gynecology, Volume 91, Issue 1, 1998, Pages 108-111, https://doi.org/10.1016/S0029-7844(97)00593-0.
- Wang T, Zhang H, Liu Y, Zhao C. Updates in Cervical Cancer Screening Guidelines, The Bethesda System for Reporting Cervical Cytology, and Clinical Management Recommendations. J Clin Transl Pathol. 2023 Jun;3(2):75-83. doi: 10.14218/jctp.2023.00004. Epub 2023 Apr 21. PMID: 37456763; PMCID: PMC10348779.
- Snijders, P. J., et al. (2013). HPV-based cervical screening: rationale, expectations and future perspectives. The Lancet Oncology, 14(10), e497-e509.
- Bennett KF, Waller J, Chorley AJ, Ferrer RA, Haddrell JB, Marlow LA. Barriers to cervical screening and interest in self-sampling among women who actively decline screening. J Med Screen. 2018 Dec;25(4):211-217. doi: 10.1177/0969141318767471. Epub 2018 Apr 13. PMID: 29649936; PMCID: PMC6262593.
- Arrivillaga M, Bermúdez PC, García-Cifuentes JP, Rodríguez-López M, Neira D, Vargas-Cardona HD. Women's critical experiences with the pap smear for the development of cervical cancer screening devices. Heliyon. 2023 Mar 7;9(3):e14289. doi: 10.1016/j.heliyon.2023.e14289. PMID: 36938419; PMCID: PMC10018556.
- Mairead O’Connor, Judith Murphy, Cara Martin, John O’Leary, Linda Sharp, the Irish Cervical Screening Consortium (CERVIVA), Motivators for women to attend cervical screening: the influential role of GPs, Family Practice, Volume 31, Issue 4, August 2014, Pages 475–482, https://doi.org/10.1093/fampra/cmu029
- Bansil, P., et al. (2021). Self-sampling for human papillomavirus (HPV) testing: A systematic review and meta-analysis. Preventive Medicine, 145, 106416.
- Baussano J, Bray F. Modelling cervical cancer elimination. Lancet Public Health 2019;4:PE23.
- Forman D, de Martel C, Lacey CJ. Global burden of human papillomavirus and related diseases.
Vaccine 2012;30S:F1223. - Renjie Wang, Wei Pan, Lei Jin, Weiming Huang, Yuehan Li, Di Wu, Chun Gao, Ding Ma, Shujie Liao, Human papillomavirus vaccine against cervical cancer: Opportunity and challenge, Cancer Letters, Volume 471, 2020, Pages 88-102, https://doi.org/10.1016/j.canlet.2019.11.039.
- Wiley DJ, et al. External genital warts: diagnosis, treatment, and prevention. Clin Infect Dis. 2002;35(Suppl 2):S210–S224.
- Perry CM, Lamb HM. Topical imiquimod: a review of its use in genital warts. Drugs. 1999;58(2):375–390.
- Godley MJ, et al. Cryotherapy compared with trichloroacetic acid in treating genital warts. Genitourin Med. 1987;63(6):390–392.
- von Krogh G, et al. European guideline for the management of anogenital warts. Int J STD AIDS. 2001;12(Suppl 3):40–47. [PubMed] [Google Scholar]
- Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, et al.. A review of human carcinogens–Part B: biological agents. Lancet Oncol. (2009) 10:321–2. 10.1016/S1470-2045(09)70096-8
- https://www.who.int/news-room/fact-sheets/detail/cervical-cancer
- Silvia de Sanjose, et. al., Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study, The Lancet Oncology, Volume 11, Issue 11, 2010, Pages 1048-1056, https://doi.org/10.1016/S1470-2045(10)70230-8.
- S.K. Kjaer, K. Frederiksen, C. Munk, T. Iftner, Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence, J. Nat. Cancer. Inst., 102 (2010), pp. 1478-1488
- Waggoner SE. Cervical cancer. Lancet 2003;361:2217–2225.
- Peto J, Gilham C, Fletcher O, Matthews FE. The cervical cancer epidemic that screening has prevented in the UK. Lancet. 2004 Jul 17-23;364(9430):249-56. doi: 10.1016/S0140-6736(04)16674-9. PMID: 15262102.
- Ojala T, Kankainen M, Castro J, Cerca N, Edelman S, Westerlund-Wikstrom B, et al. Comparative Genomics of Lactobacillus Crispatus Suggests Novel Mechanisms for the Competitive Exclusion of Gardnerella Vaginalis. BMC Genomics (2014) 15:1070. doi: 10.1186/1471-2164-15-1070
- Liang Y, Chen M, Qin L, Wan B, Wang H. A Meta-Analysis of the Relationship Between Vaginal Microecology, Human Papillomavirus Infection and Cervical Intraepithelial Neoplasia. Infect Agent Cancer (2019) 14:29. doi: 10.1186/s13027-019-0243-8
- Gur C, Ibrahim Y, Isaacson B, Yamin R, Abed J, Gamliel M, et al. Binding of the Fap2 Protein of Fusobacterium Nucleatum to Human Inhibitory Receptor TIGIT Protects Tumors From Immune Cell Attack. Immunity (2015) 42(2):344–55. doi: 10.1016/j.immuni.2015.01.010
- Engberts MK, Verbruggen BS, Boon ME, van Haaften M, Heintz AP. Candida and Dysbacteriosis: A Cytologic, Population-Based Study of 100,605 Asymptomatic Women Concerning Cervical Carcinogenesis. Cancer (2007) 111(5):269–74. doi: 10.1002/cncr.22947
Coleman HN, Greenfield WW, Stratton SL, Vaughn R, Kieber A, Moerman- Herzog AM, et al. Human Papillomavirus Type 16 Viral Load Is Decreased Following a Therapeutic Vaccination. Cancer Immunol Immunother (2016) 65(5):563–73. doi: 10.1007/s00262-016-1821-x
Kim KH, Horn TD, Pharis J, Kincannon J, Jones R, O’Bryan K, et al. Phase 1 Clinical Trial of Intralesional Injection of Candida Antigen for the Treatment of Warts. Arch Dermatol (2010) 146(12):1431–3. doi: 10.1001/ archdermatol.2010.350
- Fleischer AB, Jr., et al. Condylomata acuminata (genital warts): patient demographics and treating physicians. Sex Transm Dis. 2001;28(11):643–647.
- Weinstock H, Berman S, Cates W., Jr. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36(1):6–10.
- Scheinfeld N, Lehman DS. An evidence-based review of medical and surgical treatments of genital warts. Dermatol Online J. 2006;12((3)):5.
- Cates W., Jr. Estimates of the incidence and prevalence of sexually transmitted diseases in the United States. American Social Health Association Panel. Sex Transm Dis. 1999;26(4 Suppl):S2–S7.
- Sanclemente G, Gill DK. Human papillomavirus molecular biology and pathogenesis. J Eur Acad Dermatol Venereol. 2002;16(3):231–240.