After considerable R&D, testing and validation, Daye is excited to unveil its flagship product, the clinically-validated Cannabinoid (CBD) Tampon.
Coated in a proprietary blend of broad spectrum hemp extract (CBD extract) and cocoa butter, the CBD Tampon is the first tampon lubricated for comfort and ease, and designed with sustainability and modern users in mind.
We believe it's important to not get ahead of where the science behind cannabinoids is today. Simultaneously, we recognise the potential cannabinoids have to advance female health.
The tampon industry has seen negligible innovation in recent decades. The lack of industry precedent, a manufacturing monopoly, and limited regulatory guidance in this product category have made the development and validation of a truly novel tampon challenging.
Where regulatory obligations are deemed lacking, Daye has sought to self-regulate, and impose our own higher standards for design, manufacturing and testing so that we can be confident that our tampons are as effective and safe as they can be.

Product Safety
After initially launching in the UK, Daye is now pursuing US market launch in the near future. Although classed as personal hygiene devices in the UK (and thus, exempt from most safety checks required of medical device manufacturers), tampons are classified as medical devices in the US and require to meet the FDA’s safety requirements.
Daye believes tampons are an under-utilised (and under-regulated) medical device, and we have independently completed FDA required biocompatibility testing for medical devices (ISO 10993). These include:
- The impact of CBD tampons on lactobacilli and other good vaginal microbiome bacteria
- Whether CBD tampons increase the risk of toxic shock syndrome (TSS)
- Irritation in a lab and animal model
- Sensitisation in an animal model
- Systemic toxicity in an animal model
- Material mediated pyrogenicity
We’ve also identified precedents pointing to the safety of our CBD extract from a genotoxicity and sub-chronic toxicity; and reproductive toxicity perspective.
Further, Daye has implemented a number of additional steps in its production flow to ensure consumer safety. These include:
- Continuous lab-testing of each batch of raw materials coming into Daye’s production facility, including fibres and CBD extract. The tests include:
- Gamma rays sterilisation of the products in their final packaging
- Production in an ISO7 certified clean room
- Production on machine parts, which are entirely made of stainless steel, and sterilised twice daily according to ISO13485 and QMS standards
The finished Daye tampon is routinely tested for performance, including:
- Absorbency
- Fibre loss
Efficacy Validation
Beyond the preclinical testing required by the FDA, Daye has completed additional validation of our product with consumers in Europe to assess the safety and usability of our products.
Initial testing with 126 consumers found that:
- Daye’s CBD Tampons were rated equivalent or better than competitor tampons (uncoated organic cotton) in terms of absorbency, ease of insertion and comfort.
- Daye’s CBD Tampons reported slightly less adverse effects during use than competitor tampons though the difference was not statistically significant (8.6% compared to 9.7%).
- Daye’s tampons showed effectiveness for menstrual pain relief, as measured through self-reported patient diaries. The pain was rated on a 10-point scale (0 = no pain, 10 = worst pain imaginable).
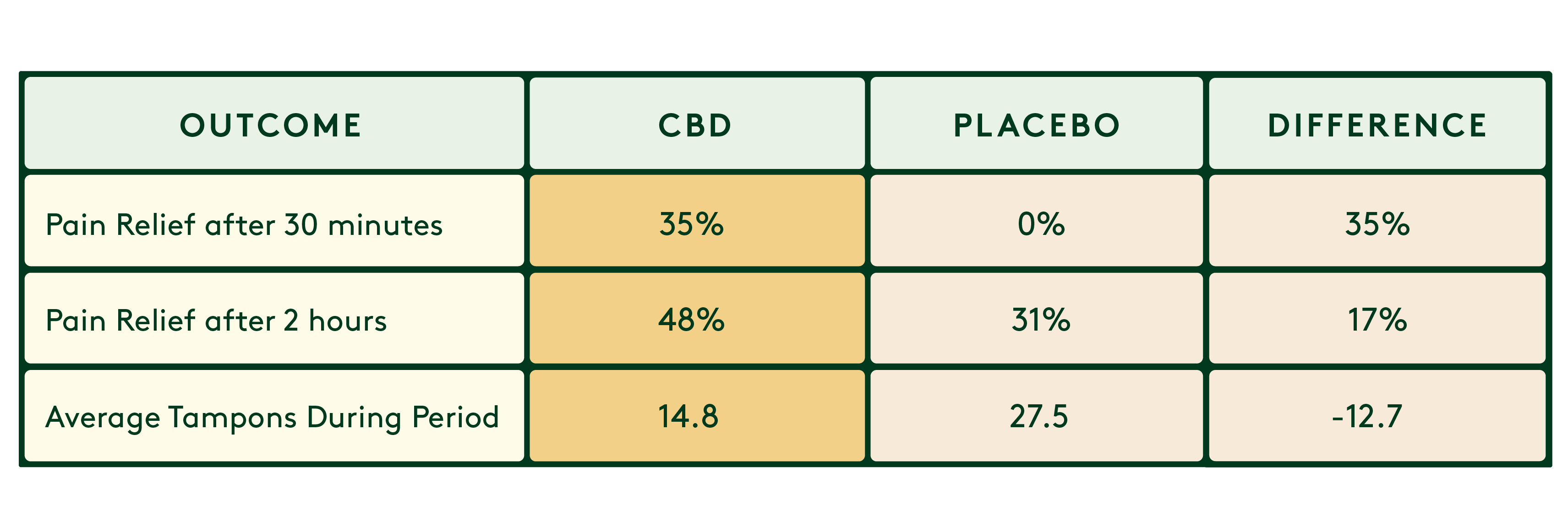
*For reference, anti-inflammatories (ibuprofen) provide 30-60% more pain relief than placebo in similar studies
Based upon this proof of concept testing, Daye has commissioned and is currently in the final stages of a placebo-controlled clinical trial to further validate the safety and efficacy of the CBD Tampon.
Although still underway, interim data analysis suggests that CBD tampons do not change the vaginal microbiome, do not cause clinically significant side effects, and show effectiveness.
Our hypothesis is that CBD tampons work by having a small percentage of the cannabinoid compound be absorbed through the vaginal mucosa, where they become active through the first uterine pass effect, and later get recycled through the pelvic organs.
The administration of medications vaginally is a relatively novel area of research and there hasn’t been much product development or innovation associated with it. However, there are a number of research papers, which demonstrate the concept’s potential:
- Miles (1994) demonstrating superior endometrial delivery of progesterone for vaginal compared with IM (despite higher plasma levels of progesterone for IM)
- Bulletti (1997) describing an ex vivo human uterine model demonstrating high endometrial concentrations of progesterone after vaginal administration
- Ziegler (1997) describing endometrial delivery of progesterone in cocoa butter vaginal suppositories with sub-physiological plasma levels
- Cicinelli (1999) describing the theoretical basis for the countercurrent diffusion mechanism for first uterine pass effect
- Krause (2010) demonstrating superior endometrial delivery of oestradiol compared to oral (vaginal oestradiol resulted in 10x higher plasma levels compared to oral, but 70x higher endometrial levels indicating preferential absorption)
- Srikrishna (2012) reviewing the feasibility of vaginal drug delivery (with reference to the first uterine pass effect)
Our thesis is that cannabidiol distributes similarly in the pelvic vasculature to steroid hormones. CBD has a molecular weight of 314 (compared to 314 for progesterone and 272 for oestradiol), and it is quite lipophilic with a logP of 6.1 (compared to 3.58 for progesterone and 3.57 for oestradiol).
- Transfer of substances is influenced by molecular size, and by the solubility of the molecules in lipids (membrane penetration)...Steroid hormones are about 250–300 Da and lipophilic; an effective transfer may thus be expected (Einer-Jensen, 2005)
- This allows a targeted local dose of CBD to the uterus, with low plasma levels of CBD in the systemic vasculature. This allows the tampon to deliver an effective dose, with minimal side effects (primarily reported in the liver and central nervous system in other CBD trials.
Post-Market Surveillance
Daye has engaged the services of an independent Clinical Advisory Board. The Board meets quarterly to review all consumer reactions reported through our post-market surveillance system. The Board’s responsibility is to continuously re-assess the clinical and patient risk associated with Daye’s CBD tampon, and to provide appropriate guidance to the company management.
Daye’s research is also vetted by an independent medical advisory board which is composed of researchers, doctors, and female health experts, who together represent some of the leading voices in reproductive health science today. The board includes (in no particular order):
- Dr. Nicola Tempest - Senior Registrar and Clinical Research Fellow, Liverpool Women’s Hospital
- Dr. Mike Armour - Leading researcher on use of cannabinoids for gynaecological pain, Western Sydney University
- Miss Claire Mellon—Consultant Obstetrician & Gynaecologist
- Prof. Louise Kenny—Executive Pro-Vice Chancellor, University of Liverpool
- Prof. Phillip Bennett—Obstetrics & Gynaecology, Imperial NHS Trust
- Prof. Douglas Kell—Research Professor of Systems Biology, University of Liverpool
- Prof. Dharani Hapangama—Professor of Obstetrics and Gynaecology, University of Liverpool
- Prof. Iain Buchan—Chair of Public Health and Clinical Informatics, University of Liverpool
- Dr. Melanie Bone—ACOG certified OBGYN
- Prof. Simon Gaisford—Professor of Pharmaceutics, University College London
- Mr. Benjamin Viaris de Lesegno—Consultant Obstetrician & Gynaecologist
Legality
The cannabinoids industry has rightly received significant scrutiny due to inconsistent chemical composition and outlandish claims. Daye has strict supplier agreements with a vertically-integrated, pharmaceutical-grade CBD extract supplier, guaranteeing that our users can be confident of the content and safety of extract used in our tampons.
Daye uses 100mg of broad-spectrum CBD extract in each coating, which consists of 30% CBD and 0% THC and is checked during rigorous batch testing by an independent laboratory.
Importantly, Daye’s CBD extract is only ever extracted from the industrial hemp plant, rather than from cannabis sativa. Industrial hemp can be legally grown and processed throughout the European Union*, and was legalised on a federal level in the US in November 2018.